A Reflection on Global Fatty Liver Day 2024: How Organ-on-a-Chip Addresses R&D Challenges
To commemorate Global Fatty Liver Day on June 13, this article explores how organ-on-a-chip models are transforming liver disease research.
Complete the form below to unlock access to ALL audio articles.
Over the last 12 months, there have been positive changes in the field of non-alcoholic steatohepatitis (NASH) research. New nomenclature has been gaining traction, with MASLD (metabolic dysfunction-associated steatotic liver disease) replacing the previously used term NAFLD (non-alcoholic fatty liver disease) to encompass a broader number of steatosis types.
Importantly, the term MASLD also helps to remove the stigma associated with the words “non-alcoholic” and “fatty”, which has been shown to result in reduced consideration in health policy and negative patient behaviors such as decreased likelihood to seek medical attention.
There is also increased hope for patients presenting with MASLD’s more advanced fibrotic form MASH (metabolic dysfunction associated steatohepatitis), a potentially fatal illness that arises from fat accumulation, inflammation and fibrosis of the liver. Until recently, there were no drug treatments approved to treat MASH; however, a significant milestone was reached in 2024, with the FDA approval of resmetirom (Rezdiffra™), an oral thyroid hormone receptor-beta (THR-β) agonist.
Challenges in MASH drug development
Despite resmetirom’s approval, many challenges remain. Firstly, the long-term safety profile of the drug is yet to be determined, as adverse effects can develop over time. Secondly, MASLD/MASH is a complex multifactorial disease that is often linked to obesity and metabolic syndromes.
Because of this, it is possible that resmetirom alone may not prove to be an effective treatment for all patients. This is a significant ongoing challenge for the healthcare sector, and one that will likely require combination therapies, targeting different aspects of the disease and extending beyond the liver to other organs, to effectively combat the disease.
Although the pipeline of drugs and drug combinations in clinical trials for MASLD/MASH has been described as “An impending Tsunami”, much remains unknown about the onset of this illness, and testing in humans is both risky and costly.
Lack of efficacy remains the leading cause of drug failures in clinical trials, so although the future looks brighter, the need for better treatment options persists. In response, however, a rising tide of research and development has been observed.
Ultimately, late-stage drug failures due to poor efficacy result from the inability of preclinical (in vitro and in vivo) models to accurately predict human outcomes. The reason for this is that their physiological relevance to humans is inherently limited.
Organ-on-a-chip models offer greater relevance
Growing demand for more predictive preclinical tools to plug the “relevance” gap has led to the development of several new approach methodologies (NAMs). Leading the alternative methodology sector are organ-on-a-chip (OOC) models, or microphysiological systems (MPS), that enable a deeper mechanistic understanding of disease, and deliver more translatable preclinical research and development insights.
OOC models utilize co-cultures of primary human cells grown on perfused scaffolds to mimic blood flow. These human organ mimics recapitulate the key microarchitecture, phenotypes and functions of healthy or diseased human organs in the laboratory.
For MASLD and MASH, the disease phenotype is induced from healthy liver-on-a-chip cultures, comprised of primary human hepatocytes, stellate and Kupffer cells, following treatment with a proprietary HEP-Fat media (Figure 1). The model can be used to study genetic polymorphisms, the mechanisms of disease and drug action.1,2
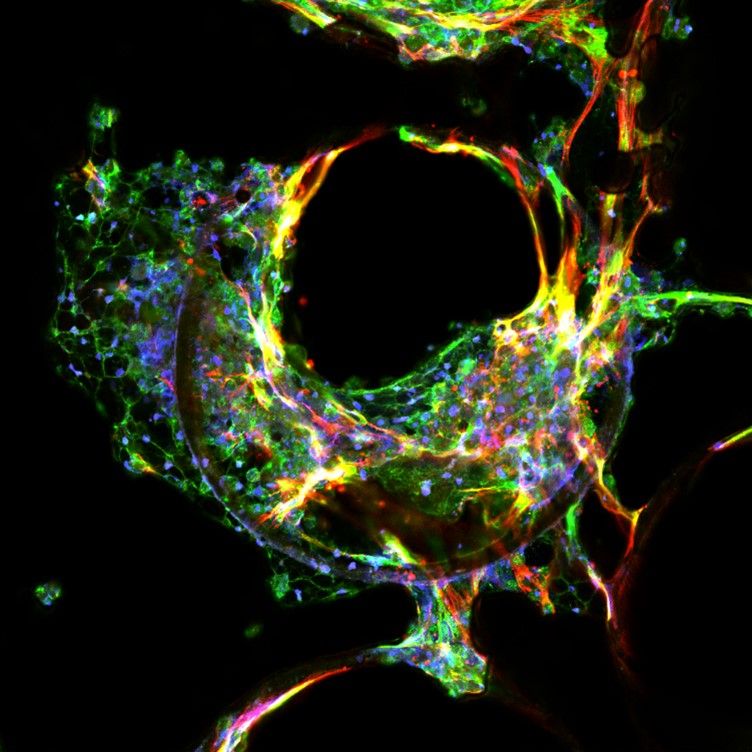
Figure 1: A confocal image of MASH liver tissue generated using MPS. Credit: CN Bio.
Physiologically-relevant mechanistic insights are generated from OOC models through the measurement of clinically relevant inflammatory, steatosis and fibrotic biomarkers associated with the MASH phenotype, providing high-content quantitative data that aids translatability between the laboratory and the clinic. Human organ MASH models have been characterized in various studies and validated using a variety of drugs, including those in recent clinical trials (Figure 2).1,2,3
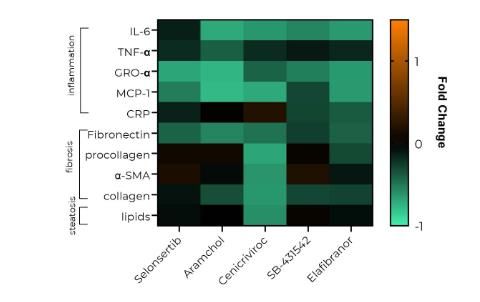
Figure 2: Efficacy screening of anti-MASH drugs using a panel of inflammatory, fibrosis and steatosis biomarkers using MPS. Credit: CN Bio.
Where animals capture the complexity of a whole organism, lab-grown organs-on-chips demonstrate how the disease mechanism, or the effects of drugs, may differ in a human setting.
In 2020, Vacca et al. explored the transcriptomic profile of this MASH model, demonstrating a strong profile compared to human data and one that more closely replicates changes found in MASH patients compared to the murine Western diet (WD) model.3 The complementary use of OOC models alongside animals, therefore, gives a broader insight into a drug’s potential in a human setting.
Murine model testing of drugs targeting MASLD/MASH is commonplace; however, their use is less suited for the development of drugs targeting human metabolism. As metabolism in mice is very different to humans, OOC models offer a viable path forward where translatability to humans is likely to be poor. Likewise, key genetic factors associated with MASH may be expressed differently, or not at all, in animal models.
In late 2023, organ-on-a-chip data supported the initiation of Inipharm’s INI-822, targeting human metabolism, into clinical trials.4 This was the first example of OOC data supporting the clinical progression of a drug for this complex metabolic disorder, demonstrating the transformative potential of the approach.
Using OOC models to predict bioavailability
In addition to studying this liver disease, or the efficacy of drugs within the liver in isolation, OOC can now link healthy or diseased organs together to mimic human processes such as first-pass metabolism and provide information regarding a drug’s bioavailability – a key parameter required for dose setting in the clinic.
Animals are notoriously poor predictors of human bioavailability. In a seminal study investigating 184 compounds, animal models were found to have a weak correlation with bioavailability in humans (R2 = 0.34).5 A new approach that is physiologically relevant to humans, which combines oral absorption and hepatic metabolism, is therefore required to provide more accurate estimations and dose setting ahead of the clinic. This approach would give drug developers greater confidence that an optimal concentration of a drug will reach its intended target, to elicit maximal efficacy whilst minimizing toxicity and decreasing the risk of late-stage failures.
Furthermore, interlinked multi-organ systems facilitate inter-organ cross talk, opening a world of future possibilities to study shared risk factors involved in MASLD/MASH, such as diabetes, obesity and metabolic syndrome. They offer the potential to explore key bidirectional axes, such as the gut–liver axis, to identify how poor diet causes dysbiosis of the gut microbiome and how this affects liver health and causes inflammation.
A deeper understanding of interactions such as these, and other influencing axes, will help to gradually unlock the unknowns of this multifaceted disease, holding promise for future treatments in years to come. But let’s not forget that drug treatments are a last resort, as most forms of MASLD and its progression into MASH are entirely preventable.
Alongside the development of new treatments, it is key that we support education efforts to improve disease awareness and promote healthy lifestyle modifications such as exercise and dietary changes. Changes such as these can prevent, halt or even reverse disease onset and remain an essential factor in curbing the disease’s growing prevalence.
About the author:
Dr. Oliver Culley is a senior scientist at CN Bio, where his work focuses on organ-on-a-chip modeling of NASH disease using the PhysioMimix OOC System. Oliver completed a BSc (Hons) Biomedical Sciences at University of Manchester and a PhD in cell biology at King's College London, before undertaking postdoctoral work in bioengineering at Queen Mary University of London. Oliver has over 10 years of experience in academia and industry with extensive experience in high-content imaging and in vitro assay design within the fields of cell and molecular biology.
1. Kostrzewski T, Maraver P, Ouro-Gnao L, et al. A microphysiological system for studying nonalcoholic steatohepatitis. Hepatol Commun. 2020;4(1):77–91. doi: 10.1002/hep4.1450
2. Kostrzewski T, Snow S, Battle AL, et al. Modelling human liver fibrosis in the context of non-alcoholic steatohepatitis using a microphysiological system. Commun Biol. 2021;4:1080. doi: 10.1038/s42003-021-02616-x
3. Vacca M, Leslie J, Virtue S, et al. Bone morphogenetic protein 8B promotes the progression of non-alcoholic steatohepatitis. Nat Metab. 2020;2:514–531. doi: 10.1038/s42255-020-0214-9
4. CN Bio. CN Bio PhysioMimix® Organ-on-a-Chip data supports Inipharm’s INI-822 for metabolic liver disease treatment now in clinical testing. https://cn-bio.com/physiomimix-data-supports-inipharms-ini-822-for-metabolic-liver-disease-treatment/. Published December 4, 2023. Accessed May 30, 2024.
5. Musther H, Olivares-Morales A, Hatley OJD, Liu B, Rostami Hodjegan A. Animal versus human oral drug bioavailability: Do they correlate? Eur J Pharm Sci. 2014;57(100):280–291. doi: 10.1016/j.ejps.2013.08.018