Product News
Bio-Rad Launches Celselect Slides 2.0 To Advance Rare Cell and Circulating Tumor Cell Enrichment for Cancer Research
New Celselect slides™ design increases efficiency of circulating tumor cell capture from liquid biopsy samples for enumeration and downstream applications.
Product News
Association for Molecular Pathology Publishes Evidence-Based Recommendations for Tumor Mutational Burden Testing
AMP published a set of evidence-based recommendations for the analytical validation and reporting of tumor mutational burden (TMB) testing as a potential predictive biomarker for immune checkpoint inhibitor (ICI) therapies.
Product News
Estonia Develops Personalized CAR-T Cell Therapy for Blood Cancer Patients
The Tartu University Hospital, North Estonia Medical Centre, and Icosagen have joined forces to develop and introduce an innovative personalized cell therapy (CAR-T cell therapy) for patients in Estonia.
Product News
OncoHost To Present Poster Demonstrating PROphet®’s Application in Multiple Cancer Indications at ASCO 2024
Plasma-based proteomic platform predicts clinical benefit from immune-checkpoint inhibitors in NSCLC, melanoma, and HPV-related cancers.
Product News
Aptamer and Timser Partner To Deliver World’s First Blood Test for Cervical Cancer
Optimer binders will be developed for use in a cervical cancer blood test. Agreement for up to £465,000 signed for Optimer development.
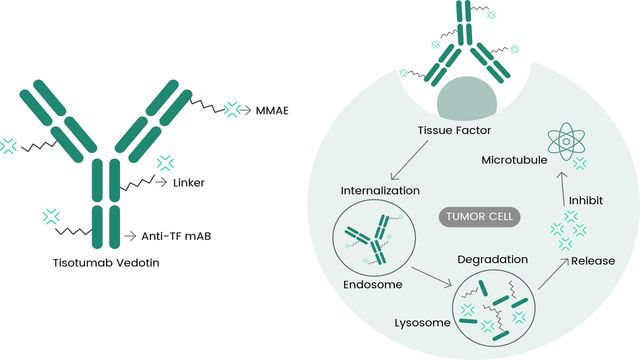
Product News
FDA-Approved TIVDAK®: Targeting Tissue Factor in Cervical Cancer
On April 29th, the U.S. FDA granted full approval for Seagen Inc.'s TIVDAK® (tisotumab vedotin) targeting tissue factor (TF) for the treatment of patients with recurrent or metastatic cervical cancer who have progressed on or after chemotherapy.
Advertisement