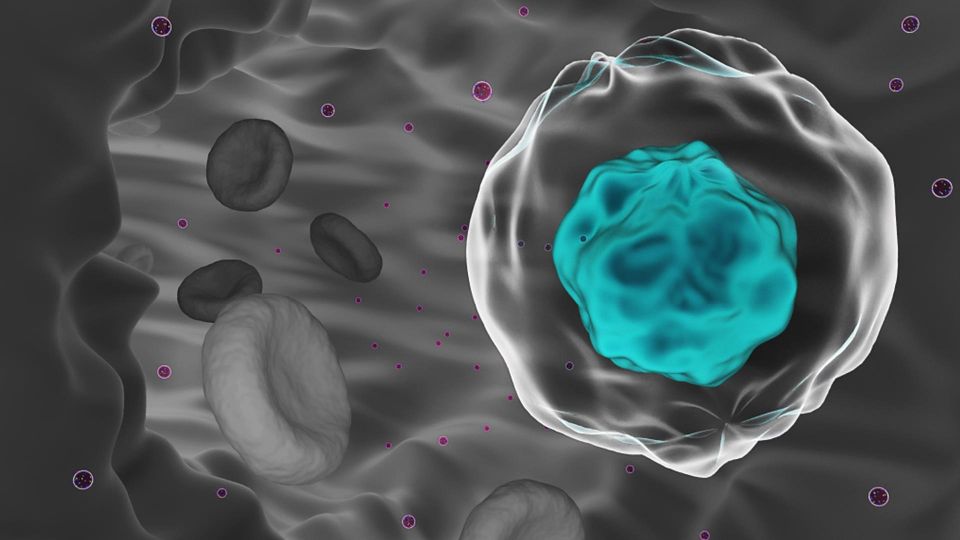
Circulating tumor cells (CTCs) arise when cancer begins to metastasize, and are increasingly being recognized as important early diagnostic and treatment markers.
However, CTCs can be challenging to isolate and analyze, due to their extreme rarity. Traditional methods of isolation and enrichment typically offer low sensitivity, limited throughput and sparse data.
Automated cell imaging and picking systems can unlock the full potential of CTCs, with rapid, automated and highly accurate methods for isolation, enrichment and identification.
Download this listicle to discover how to:
- Minimize cell damage and contamination
- Enhance selectivity and accuracy
- Increase time-efficiency with automated workflows
5 Innovative Ways to Advance Your
Circulating Tumor Cell Research
June 2024
Introduction
Metastasis accounts for ~90% of cancer-related deaths and can occur quickly after onset of disease in patients
with aggressive tumors.1,2 Metastasis arises when malignant cells slough off the primary or metastatic tumor,
.1,2
intravasate into the circulatory system and establish elsewhere in the body. These circulating tumor cells (CTCs)
are being increasingly recognized for their importance in diagnostics and treatment.
3
The ability to accurately and rapidly identify CTCs as an early marker of aggressive or metastatic cancers could be a
significant leap forward in improving cancer care. As CTCs tend to be genetically representative of the main tumor
mass, they can be used to monitor disease progression and guide treatment in a non-invasive manner.4 In addition
to clinical roles, CTCs have the potential to be cultured ex vivo for research applications, including the creation of
tumor cell lines to investigate cellular mechanisms and the efficacy of novel treatments.5
Populations of CTCs may comprise as few as one cell per 106–109 normal blood cells. Therefore despite their clinical
and research significance, they are extremely difficult to isolate and analyze.6,7 There are several traditional methods
6,7
of isolation, enrichment and analysis including the epithelial cell adhesion molecule (EpCAM)-based technique
and antigen selection methods, fluorescence-activated cell sorting (FACS) and microfluidic-based methods.
6,8
However, these approaches often offer low sensitivity, have limited in throughput, are time-consuming and may
produce limited data.